-
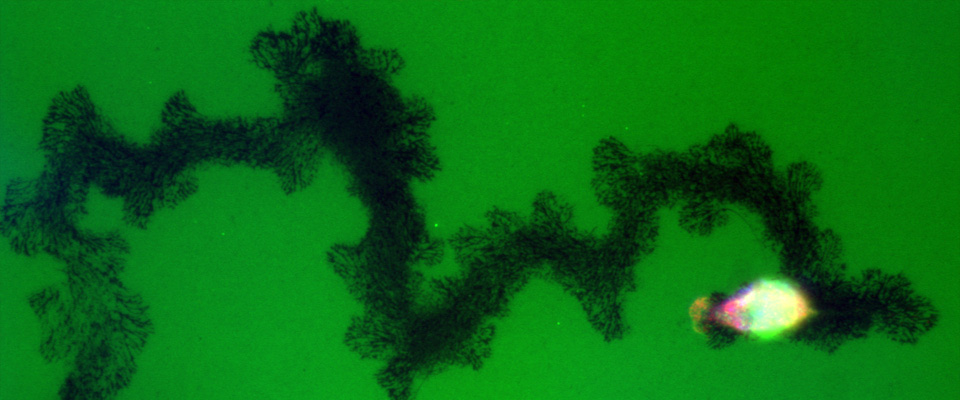
Pancreatic tumor cells grow and invade during metastasis
Degradation of the extracellular matrix during metastasis is a necessary step in cancer progression. Here, a pancreatic cancer cell is degrading a fluorescent matrix (green) as it migrates. The dark areas represent the path of the cell.
Tumor Cell Growth and Migration
Cancers of the lung, pancreas and liver are listed as the first, third and fourth most lethal cancers in the United States and are increasing rapidly, according to the National Cancer Institute. The five-year survival rate for people with these tumors is exceptionally poor due to a lack of effective therapies that target the cells of these cancers, which tend to migrate away from the site of the primary tumor to invade distal organs. This metastatic behavior of tumor cells is the single most important factor affecting survival as 90% of mortality is due to metastatic invasion. It is regulated by hundreds of different cytoskeletal-membrane proteins.
The Membrane Trafficking in Disease Lab's research team seeks to understand how these tumor cells:
- Grow, multiply and dissociate from the primary tumor.
- Remodel the surrounding tumor stroma by protease secretion to escape into adjacent blood vessels.
- Support collective and independent migration to distal locations where they can form new tumors.
The lab's investigators (in collaboration with the Cell Biology of Metastasis Lab) explore how the metastatic behavior of tumor cells is regulated to discover new treatments for lung, liver and pancreatic cancers.
Selected publications
- Gutierrez-Ruiz OL, Johnson KM, Krueger EW, Nooren RE, Cruz-Reyes N, Heppelmann CJ, Hogenson TL, Fernandez-Zapico ME, McNiven MA, Razidlo GL. Ectopic expression of DOCK8 regulates lysosome-mediated pancreatic tumor cell invasion. Cell Reports. 2023; doi:10.1016/j.celrep.2023.113042.
- Burton KM, Johnson KM, Krueger EW, Razidlo GL, McNiven MA. Distinct forms of the actin cross-linking protein α-actinin support macropinosome internalization and trafficking. Molecular Biology of the Cell. 2021; doi:10.1091/mbc.E20-12-0755.
- Cao H, Qiang L, Chen J, Johnson KM, McNiven MA, Razidlo GL. Synergistic metalloproteinase-based remodeling of matrix by pancreatic tumor and stromal cells. PLoS One. 2021; doi: 10.1371/journal.pone.0248111.
- Burton KM, Cao H, Chen J, Qiang L, Krueger EW, Johnson KM, Bamlet WR, Zhang L, McNiven MA, Razidlo GL. Dynamin 2 interacts with α-actinin 4 to drive tumor cell invasion. Molecular Biology of the Cell. 2020; doi:10.1091/mbc.E19-07-0395.
- Qiang L, Cao H, Chen J, Weller SG, Krueger EW, Zhang L, Razidlo GL, McNiven MA. Pancreatic tumor cell metastasis is restricted by MT1-MMP binding protein MTCBP-1. Journal of Cell Biology. 2019; doi:10.1083/jcb.201802032.
- Razidlo GL, Burton KM, McNiven MA. Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. Journal of Biological Chemistry. 2018; doi:10.1074/jbc.RA118.003276.
- Cao H, Eppinga RD, Razidlo GL, Krueger EW, Chen J, Qiang L, McNiven MA. Stromal fibroblasts facilitate cancer cell invasion by a novel invadopodia-independent matrix degradation process. Oncogene. 2016; doi:10.1038/onc.2015.163.
- Razidlo GL, Magnine C, Sletten AC, Hurley RM, Almada LL, Fernandez-Zapico ME, Ji B, McNiven MA. Targeting pancreatic cancer metastasis by inhibition of Vav1, a driver of tumor cell invasion. Cancer Research. 2015; doi:10.1158/0008-5472.CAN-14-3103.
- Razidlo GL, Schroeder B, Chen J, Billadeau DD, McNiven MA. Vav1 as a central regulator of invadopodia assembly. Current Biology. 2014; doi:10.1016/j.cub.2013.11.013.
- Razidlo GL, Wang Y, Chen J, Krueger EW, Billadeau DD, McNiven MA. Dynamin 2 potentiates invasive migration of pancreatic tumor cells through stabilization of the Rac1 GEF Vav1. Developmental Cell. 2013; doi:10.1016/j.devcel.2013.02.010.
- Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. Journal of Cell Biology. 2012; doi:10.1083/jcb.201105153.
- Orlichenko L, Weller SG, Cao H, Krueger EW, Awoniyi M, Beznoussenko G, Buccione R, McNiven MA. Caveolae mediate growth factor-induced disassembly of adherens junctions to support tumor cell dissociation. Molecular Biology of the Cell. 2009; doi: 10.1091/mbc.e08-10-1043.