 X-ray crystallography
X-ray crystallography
Mayo Clinic's Structural Biology Core makes X-ray crystallography equipment accessible to investigators.
X-ray crystallography
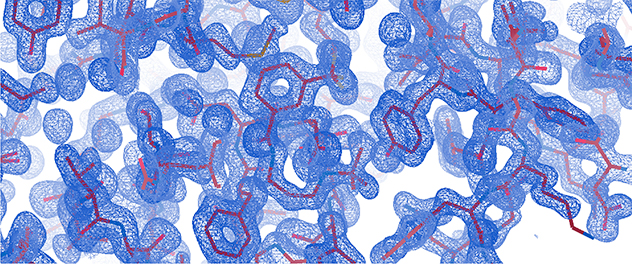
X-ray crystallography is a powerful technique for determining the 3D structures of biological macromolecules such as proteins and nucleic acids and their complexes with other macromolecules, ligands, substrates or inhibitors.
The crystallography process involves several key steps:
- Produce samples. Before crystallization can begin, researchers must obtain pure macromolecular samples. The best samples for crystallization are homogeneous in content, chemistry and conformation.
- Obtain crystals. Crystallization is often the most challenging step in X-ray crystallography. Single, near-perfect crystals are required for X-ray diffraction and structure determination.
- Collect data. After selecting and preparing crystals, data are collected using an X-ray diffractometer.
- Analyze data. After collecting diffraction data, the information is processed to create electron density maps. Then these maps are used to build and refine detailed atomic models of the crystal structure.
The Structural Biology Core provides access to these instruments:
- Rigaku XtaLAB Synergy-R diffractometer for X-ray crystallography. The system includes a PhotonJet-R microfocus rotating anode X-ray source, a curved HyPix-Arc 150-degree detector and a Cobra cooling system from Oxford Cryosystems Ltd.
- Mosquito crystallization robot from SPT Labtech. The Mosquito robot automates crystallization by dispensing nanoliter volumes with high precision.