-
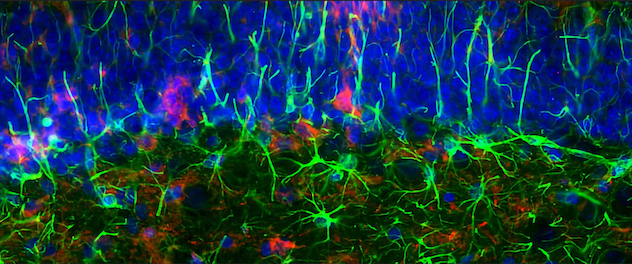
Tau propagation animal model to understand disease mechanisms
Dr. Ikezu strives to find disease mechanisms by using animal models that recapitulate tau pathology found in Alzheimer's disease and tauopathy in patients' brains. The image captures the accumulation of pathological tau in the CA1 hippocampal region of a mouse brain. Activated glial cells surround this tau.
-
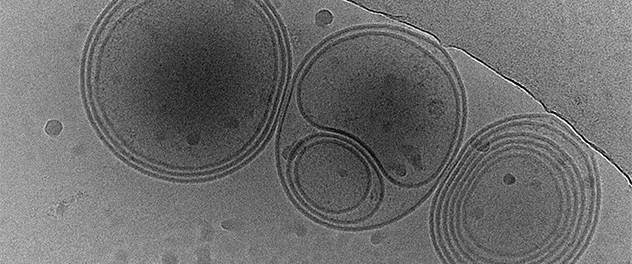
Extracellular vesicles are vehicles to transfer physiological and pathological molecules between cells
Dr. Ikezu's team investigates nanoscale particles exchanged between the cells as carriers of pathological tau protein. Cryoelectron microscopy captures the multiple membrane structure of extracellular vesicles isolated from human brain tissues.
-
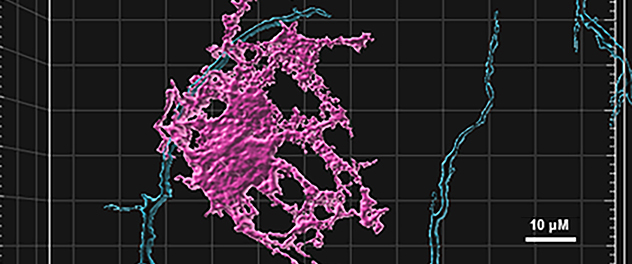
Human iPSC-derived brain cells as a disease model in a dish
We take advantage of the opportunity to use patient-derived induced pluripotent stem cells (iPSCs) established on Mayo Clinic's Florida campus to understand disease mechanisms and develop a drug to treat patients with Alzheimer's disease and tauopathies. The image captures human iPSC-derived neurons (blue) and oligodendrocytes (magenta) interacting for myelination in vitro.
Overview
Mayo Clinic's Molecular Neurotherapeutics Laboratory, led by Tsuneya Ikezu, M.D., Ph.D., identifies target molecules and cell types and develops therapies to treat or prevent neurodegenerative conditions. These conditions include Alzheimer's disease and other tauopathies, such as frontotemporal lobar degeneration, Lewy body dementia, corticobasal degeneration and progressive supranuclear palsy.
Neurodegenerative disorders
The primary focus of Dr. Ikezu's lab is to understand how tau protein propagates in a stereotypical manner in the brain of patients with Alzheimer's disease and tauopathies. Dr. Ikezu's team identified that microglia and extracellular vesicles mediate the spread of pathogenic tau protein in animal models of Alzheimer's disease.
The team also characterizes lipidomic and proteomic profiles of extracellular vesicles isolated from human brain tissues and biofluids to determine their role in disease spread, intercellular communications and biomarker discovery. This work is done in collaboration with investigators who have expertise in advanced mass spectrometry.
Human-induced pluripotent stem cell (iPSC) research
Human iPSCs are powerful tools for reconstituting the interaction of human neuronal and immune cells in tissue culture models. We are remodeling the central nervous system (spheroids), which consists of many neuronal cell types, using human iPSCs derived from people with illness and those without. These models are being tested using confocal microscopy, electrophysiology, RNA sequencing and proteomics. Moreover, we perform CRISPR editing of the iPSC genome and differentiate human microglia or oligodendrocytes that are adapted to survive in neurodegenerative conditions. The goal is to develop next-generation biotherapeutics.
Patient resources