 GSK3β is a major hub in MASH mice LSECs
GSK3β is a major hub in MASH mice LSECs
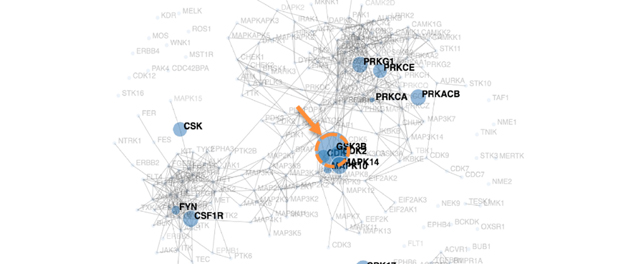
A kinome map was generated from liver sinusoidal endothelial cell (LSEC) phospho-proteomics. The orange arrow and dotted circle point to glycogen synthase kinase (GSK) 3β as the major hub in primary mouse LSEC on a metabolic dysfunction-associated steatohepatitis (MASH)-inducing diet.