-
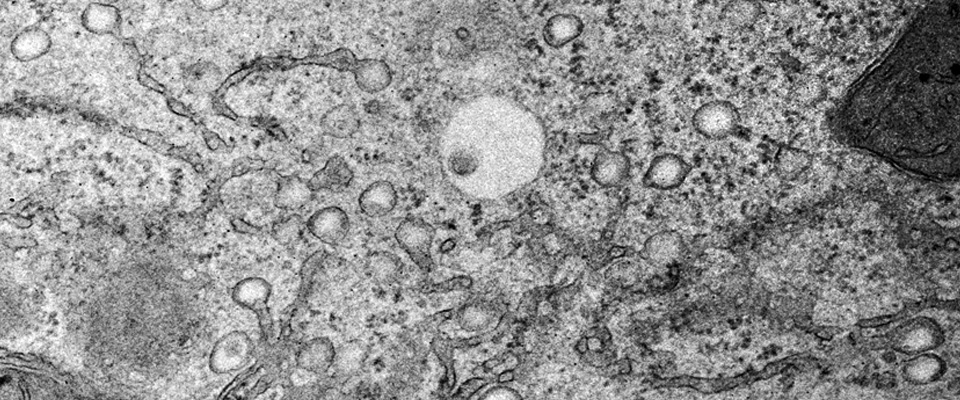
Lipid drop formation at the endoplasmic reticulum is crucial for proper fat storage
Electron micrograph of a primary rat hepatocyte shows the formation of nascent lipid drops from the endoplasmic reticulum.
Lipid Droplet Biology and Fatty Liver
Fatty liver disease, also called hepatic steatosis, has become the most common disease of the liver in the United States. Over 40% of Americans have a fatty liver that can lead to liver fibrosis, inflammation (hepatitis), cirrhosis and cancer. Moreover, because the liver is a central detoxification organ, it is especially susceptible to damage from alcohol. Most heavy drinkers exhibit fatty liver, called ethanol-induced hepatic steatosis, and 40% to 50% of these people can develop life-threatening disease. The central organelle within the hepatocyte that stores excess fat is the lipid droplet (LD). Scores of different proteins control the formation and use of LDs. A deficit in LD breakdown is believed to play a central role in fatty liver disease.
The Membrane Trafficking in Disease Lab's goals are to define:
- How the hepatocyte targets different catabolic proteins to the LD surface to mediate LD breakdown.
- The mechanisms by which autophago-lysosomes engulf LDs in a process termed "lipophagy" to mediate catabolism.
- The mechanisms by which LDs-autophago-lysosomes-mitochondria interact to expedite lipid catabolism.
- How alcohol alters LD composition, function and breakdown.
- How alcohol alters the association of LD-bound proteins that can contribute to fat catabolism and autophagy.
Selected publications
- Thomes PG, Strupp MS, Donohue TM Jr, Kubik JL, Sweeney S, Mahmud R, Schott MB, Schulze RJ, McNiven MA, Casey CA. Hydroxysteroid 17β-dehydrogenase 11 accumulation on lipid droplets promotes ethanol-induced cellular steatosis. Journal of Biological Chemistry. 2023; doi:10.1016/j.jbc.2023.103071.
- Schulze RJ, Krueger EW, Weller SG, Johnson KM, Casey CA, Schott MB, McNiven MA. Direct lysosome-based autophagy of lipid droplets in hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 2020; doi:10.1073/pnas.2011442117.
- Drizyte-Miller K, Chen J, Cao H, Schott MB, McNiven MA. The small GTPase Rab32 resides on lysosomes to regulate mTORC1 signaling. Journal of Cell Science. 2020; doi:10.1242/jcs.236661.
- Li Z, Weller SG, Drizyte-Miller K, Chen J, Krueger EW, Mehall B, Casey CA, Cao H, McNiven MA. Maturation of lipophagic organelles in hepatocytes is dependent upon a Rab10/Dynamin-2 complex. Hepatology. 2020; doi:10.1002/hep.31059.
- Schott MB, Weller SG, Schulze RJ, Krueger EW, Drizyte-Miller K, Casey CA, McNiven MA. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. Journal of Cell Biology. 2019; doi:10.1083/jcb.201803153.
- Schulze RJ, Rasineni K, Weller SG, Schott MB, Schroeder B, Casey CA, McNiven MA. Ethanol exposure inhibits hepatocyte lipophagy by inactivating the small guanosine triphosphatase Rab7. Hepatology Communications. 2017; doi:10.1002/hep4.1021.
- Schott MB, Rasineni K, Weller SG, Schulze RJ, Sletten AC, Casey CA, McNiven MA. β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. Journal of Biological Chemistry. 2017; doi:10.1074/jbc.M117.777748.
- Li Z, Schulze RJ, Weller SG, Krueger EW, Schott MB, Zhang X, Casey CA, Liu J, Stöckli J, James DE, McNiven MA. A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets. Science Advances. 2016; doi:10.1126/sciadv.1601470.
- Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015; doi:10.1002/hep.27667.
- Schulze RJ, Weller SG, Schroeder B, Krueger EW, Chi S, Casey CA, McNiven MA. Lipid droplet breakdown requires dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes. Journal of Cell Biology. 2013; doi:10.1083/jcb.201306140.