-
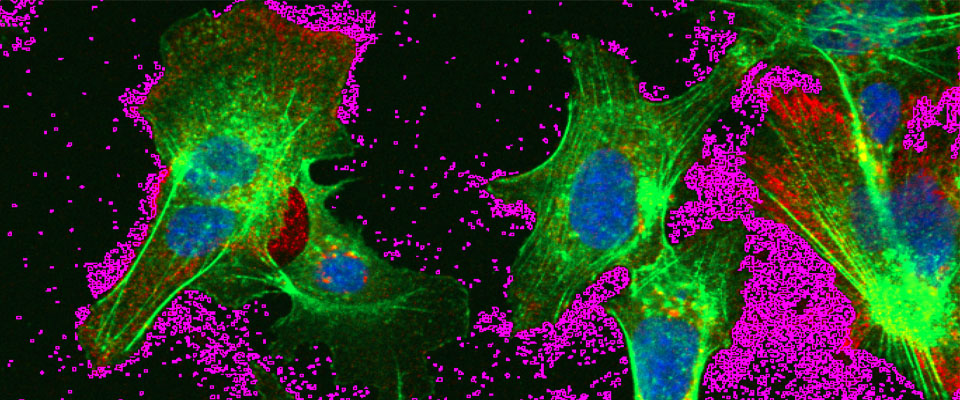
Hepatocytes infected with hepatitis B virus
Hepatocytes infected with hepatitis B virus secrete large numbers of exosomes, highlighted in purple. These exosomes also can contain hepatitis B virions.
Mechanisms of Hepatitis B Infection
Hepatitis B virus (HBV) infection is a global health problem because individuals infected with the virus are at risk of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. It is estimated that 350 to 400 million people have chronic HBV infection despite the availability of effective vaccines. Thus, a better understanding of the HBV life cycle and the mechanisms by which this virus usurps the established vesicle trafficking, autophagic, secretory and endocytic pathways of the hepatocyte host is essential to enable the identification of new antiviral targets.
Selected publications
- Ninomiya M, Inoue J, Krueger EW, Chen J, Cao H, Masamune A, McNiven MA. The exosome-associated tetraspanin CD63 contributes to the efficient assembly and infectivity of the hepatitis B virus. Hepatology Communications. 2021; doi:10.1002/hep4.1709.
- Inoue J, Krueger EW, Chen J, Cao H, Ninomiya M, McNiven MA. HBV secretion is regulated through the activation of endocytic and autophagic compartments mediated by Rab7 stimulation. Journal of Cell Science. 2015; doi:10.1242/jcs.158097.
- Abdulkarim AS, Cao H, Huang B, McNiven MA. The large GTPase dynamin is required for hepatitis B virus protein secretion from hepatocytes. Journal of Hepatology. 2003; doi:10.1016/s0168-8278(02)00326-4.
- Accola MA, Huang B, Al Masri A, McNiven MA. The antiviral dynamin family member, MxA, tubulates lipids and localizes to the smooth endoplasmic reticulum. Journal of Biological Chemistry. 2002; doi:10.1074/jbc.M201641200.