Tissue Repair and Regeneration: Mechanisms Underlying Cellular Senescence and Acute Skeletal Injury
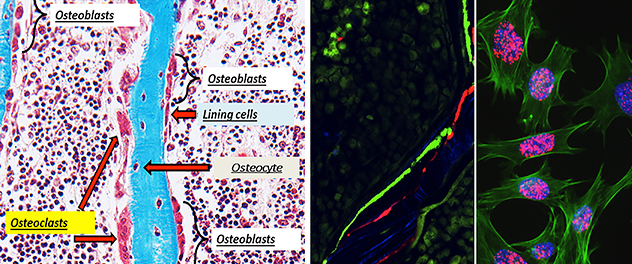 Bone tissue regeneration: A cellular milieu
Bone tissue regeneration: A cellular milieu
Targeting bone tissue regeneration with therapeutics requires a proper understanding of underlying cellular mechanisms. The Bone Injury and Repair Lab deciphers these mechanisms to determine how they affect bone remodeling in various disease conditions.
Radiotherapy with or without chemotherapy has successfully treated people with cancer. With increased survivorship, the bystander effects of radiotherapy, including bone damage, result in a gamut of bone changes. These changes include osteopenia, osteoporosis, osteoradionecrosis and fractures.
Older adult populations are predisposed to osteoporotic fractures and are at a higher risk of bone damage from radiotherapy. Dr. Chandra's lab is working to develop a viable therapeutic intervention that alleviates radiotherapy-induced osteoporosis.
Radiotherapy is modeled in animals through focal radiation therapy. We have previously shown that, in part, DNA damage and cellular apoptosis cause radiation damage to rodent bone, thereby reducing functional osteoblasts and osteocytes. These cellular changes do not account for more than 90% suppression in bone formation rate. Dr. Chandra's research team reduced radiation-induced bone damage and cellular apoptosis and improved bone formation. They did so using bone anabolic drugs, such as parathyroid hormone and neutralizing antibodies against sclerostin, and by stabilizing DNA repair proteins using proteasome inhibitors.
Dr. Chandra's Bone Injury and Repair Lab also investigates the role of senescent cells and their secretome in regulating acute skeletal injury and repair. Cellular senescence — one of the major pathways induced following radiotherapy-related DNA damage — is characterized by a proinflammatory, senescence-associated secretory phenotype consisting of chemokines, cytokines, growth factors, matrix degrading enzyme and other molecules. Cyclin-dependent kinase inhibitors p16Ink4a and p21Cip1 mainly regulate cellular senescence.
Until recently, it was understood that p16Ink4a and p21Cip1 were coexpressed in all senescent cells and they regulated their functions interchangeably. But this theory did not match with the expression pattern of p16Ink4a and p21Cip1 following radiotherapy or in aging.
Using gene expression and RNA in situ hybridization studies, we have shown that cells express p21 or p16Ink4a in unique populations of bone marrow cells, osteoblasts and osteocytes, essentially independent of the expression of other senescence markers. Only a small proportion of cells express both p16Ink4a and p21Cip1.
We identified that radiotherapy-induced injury to bone is a p21-dependent process. Using genetic clearance of senescent cells in p21-ATTAC mice, we showed significant rescue of bone damage from acute senescence generated by 24 Gy focal radiation treatment.
Mesenchymal stem cells are more resistant to DNA damage and apoptosis. They can transdifferentiate into adipocytes after radiotherapy, leading to increased bone marrow adiposity.
Dr. Chandra's lab and others have shown that focal radiation therapy-induced bone marrow adiposity occurs at the expense of the total mesenchymal stem cell population. But whether increases in the senescence burden drives bone marrow adiposity has not yet been established. Moreover, the relationships among bone marrow adiposity and proinflammatory factors such as senescence-associated secretory phenotype — factors that are abundant in a senescent bone marrow environment — have not been determined. Our goal is to provide viable therapeutics to alleviate radiotherapy-related bone deterioration and improve quality of life for people who have been treated for cancer.
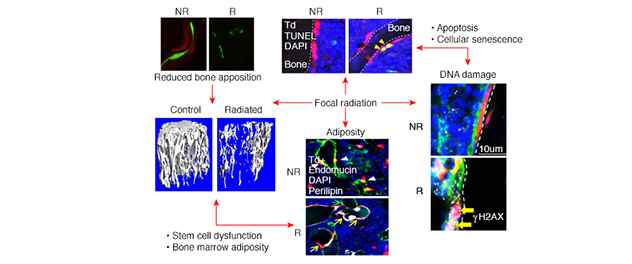 Radiotherapy-related bone damage: A multifaceted condition
Radiotherapy-related bone damage: A multifaceted condition
Bone is a dynamic organ with distinguished cellular functions affecting overall bone quality. Understanding mechanisms underlying these cellular-to-functional changes in bone after an extreme condition such as radiation enables the Bone Injury and Repair Lab to propel therapeutic outcomes.
Recent publications
- Chandra A, Lagnado AB, Farr JN, Doolittle M, Tchkonia T, Kirkland JL, LeBrasseur NK, Robbins PD, Niedernhofer LJ, Ikeno Y, Passos JF, Monroe DG, Pignolo RJ, Khosla S. Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell. 2022; doi:10.1111/acel.13602.
- Chandra A, Lagnado AB, Farr JN, Schleusner M, Monroe DG, Saul D, Passos JF, Khosla S, Pignolo RJ. Bone marrow adiposity in models of radiation- and aging-related bone loss is dependent on cellular senescence. Journal of Bone and Mineral Research. 2022; doi:10.1002/jbmr.4537.
- Chandra A, Lagnado AB, Farr JN, Monroe DG, Park S, Hachfeld C, Tchkonia T, Kirkland JL, Khosla S, Passos JF, Pignolo RJ. Targeted reduction of senescent cell burden alleviates focal radiotherapy-related bone loss. Journal of Bone and Mineral Research. 2020; doi:10.1002/jbmr.3978.
- Pignolo RJ, Samsonraj RM, Law SF, Wang H, Chandra A. Targeting cell senescence for the treatment of age-related bone loss. Current Osteoporosis Reports. 2019; doi:10.1007/s11914-019-00504-2.