Mesenchymal Stem Cell Dysfunction During Aging and Disease
 Cellular fate of bone marrow-derived mesenchymal stem cells
Cellular fate of bone marrow-derived mesenchymal stem cells
Mesenchymal stem cells are multipotent stem cells found in bone marrow. Mesenchymal stem cells are important for making and repairing skeletal tissues, such as cartilage, bone and the fat found in bone marrow. With age and disease, mesenchymal stem cells predominantly convert into lipid-accumulating fat cells. This reduces the overall pool of these cells in the bone marrow reservoir, affecting future bone formation. Dr. Chandra's lab focuses on understanding the genetic and molecular triggers that drive cell fate conversion to regulate them therapeutically.
Mesenchymal stem cells are multipotent stem cells that can separate into various cell types, including:
- Bone cells, called osteoblasts.
- Cartilage cells, termed chondrocytes.
- Muscle cells, called myocytes.
- Fat cells that give rise to marrow adipose tissue, called adipocytes.
Bone marrow has a limited mesenchymal stem cell pool that depletes with age and after disease. Intriguingly, depletion in functional mesenchymal stem cells often correlates with an increase in adiposity. This is one major reason bones fail to regenerate in osteoporotic conditions.
Dr. Chandra focuses on therapies to regulate bone marrow adiposity. His research aims to define the cues that regulate mesenchymal cell fate and assign them to an osteogenic or adipogenic fate.
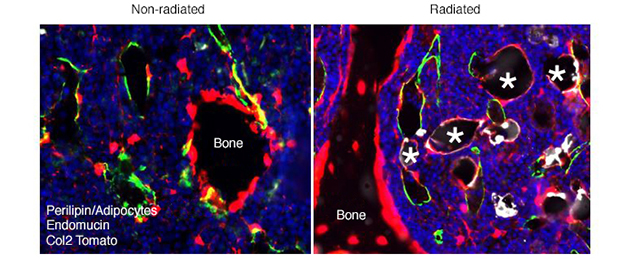
Dr. Chandra uses lineage-tracing methods to accurately characterize mesenchymal fate and identify the pathways that regulate cell fate. He has used age- and radiation-related bone marrow adiposity in vivo and in vitro to determine whether senescence plays a role in mesenchymal stem cell depletion due to fate conversion to adipocytes. A multidrug screening is identifying compounds that can regulate this process and may be potential agents to treat osteoporosis.
Recent publications
- Chandra A, Lagnado AB, Farr JN, Doolittle M, Tchkonia T, Kirkland JL, LeBrasseur NK, Robbins PD, Niedernhofer LJ, Ikeno Y, Passos JF, Monroe DG, Pignolo RJ, Khosla S.
Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell. 2022; doi:10.1111/acel.13602.
- Chandra A, Lagnado AB, Farr JN, Schleusner M, Monroe DG, Saul D, Passos JF, Khosla S, Pignolo RJ. Bone marrow adiposity in models of radiation- and aging-related bone loss is dependent on cellular senescence.
Journal of Bone and Mineral Research. 2022; doi:10.1002/jbmr.4537.