Human developmental disorders arising from mutations in epigenetic regulator genes
 Epigenetic changes during stem cell differentiation
Epigenetic changes during stem cell differentiation
Understanding epigenetic changes that regulate cellular differentiation is key to understanding how cancers and developmental syndromes arise and how they can be more effectively treated.
Disruption of epigenetic regulator genes in cancer is common. This disruption typically manifests as the loss of both gene copies in somatic cells. But single copy losses of many of these same epigenetic regulators during development leads to a group of developmental syndromes.
These developmental syndromes have been termed Mendelian disorders of the epigenetic machinery (MDEMs), chromatinopathies or overgrowth with intellectual disability disorders. Although rare individually, MDEMs collectively account for up to 19% of intellectual disability diagnoses and 45% of intellectual disability accompanied by overgrowth diagnoses out of an estimated 2 to 5 million U.S. children exhibiting abnormal growth.
Characteristic features of many MDEMs include growth abnormality — overgrowth or undergrowth — and phenotypes related to intellectual disability or autism. These features link to monoallelic mutations or microdeletions in chromatin regulatory genes.
Treatments for these rare diseases remain elusive. Several MDEMs converge on pathways linked to histone H3 lysine 36 methylation (H3K36me), including:
- Sotos syndrome. NSD1, which writes H3K36me1/2, mutates in Sotos syndrome.
- Tatton-Brown-Rahman syndrome. DNMT3A, which writes DNA methylation and is targeted by H3K36me, mutates in this disease.
- Weaver syndrome. EZH2, which writes H3K27me and is targeted by H3K36me, mutates in this disease.
- Luscan-Lumish syndrome. SETD2, which writes H3K36me3 and directs DNMT3B localization, mutates in Luscan-Lumish syndrome.
How haploinsufficiency in chromatin regulators disrupts neurodevelopmental processes remains largely unknown.
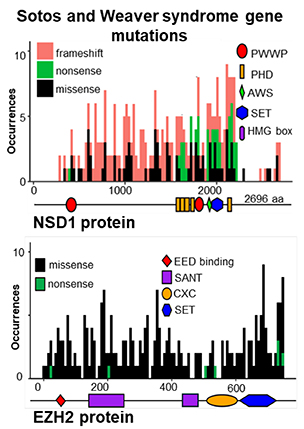 Summary of mutations in the NSD1 gene (top) and the EZH2 gene (bottom) in Sotos syndrome and Weaver syndrome, respectively.
Summary of mutations in the NSD1 gene (top) and the EZH2 gene (bottom) in Sotos syndrome and Weaver syndrome, respectively.
This is a frequency plot of mutations found in the NSD1 and EZH2 genes in people with Sotos syndrome and Weaver syndrome, respectively. The overall structure of each protein is shown as a horizontal line below each plot. The location of key functional domains also is shown. PWWP stands for proline-tryptophan-tryptophan-proline. PHD stands for plant homeodomain. AWS stands for associated with SET. SET indicates the methyltransferase catalytic domain. HMG stands for high-mobility group domain. EED stands for embryonic ectoderm development domain. SANT stands for the Swi3, Ada2, N-Cor and TFIIIB domain. CXC stands for cysteine-rich domain.
Dr. Robertson's Epigenetic Etiology of Human Disease Laboratory is studying these rare diseases by developing patient-derived induced pluripotent stem cell (iPSC) models, along with normal iPSC lines, engineered to express patient-derived mutations. The lab is using a combination of epigenome profiling studies and directed differentiations to understand how mutations in epigenetic regulators give rise to developmental syndromes and how new therapies can be developed to treat people affected by these rare diseases.
Another rare human disease linked to mutation of epigenetic regulators is immunodeficiency, centromeric instability and facial anomalies (ICF) syndrome. Mutations in DNMT3B, ZBTB24, CDCA7, HELLS and possibly other yet-to-be-identified epigenetic regulator genes cause ICF syndrome. This rare disorder is characterized by immune alterations primarily impacting B cell development, as well as facial anomalies and profound genomic instability involving the centromeric regions of chromosomes 1, 9 and 16.
Mutations in the DNMTs have been identified in human genetic diseases and cancer. As such, researchers in the Epigenetic Etiology of Human Disease Laboratory expect that studying ICF syndrome will generate novel information on the role of DNA methylation in health and disease states in general. In addition, these studies may yield new treatments for ICF syndrome or diseases with alterations in regulation of genomic methylation patterns.