Biological mechanisms of peripheral neuropathy
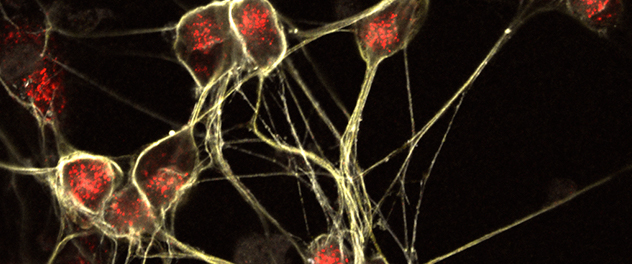
Human sensory neurons derived from induced pluripotent stem cells (iPSCs).
To develop treatments and neuroprotection for peripheral neuropathy, the Translational Neuromuscular Disease Research Lab at Mayo Clinic has several active studies that are investigating the basic biological mechanisms of peripheral neuropathy. The primary laboratory model is in vitro human sensory neurons. These sensory neurons are manufactured from human skin biopsy-derived induced pluripotent stem cells (iPSCs). Human neurons allow the laboratory to take advantage of human biology and study the specific human cells affected in a variety of diseases.
Chemotherapy-induced peripheral neuropathy
Chemotherapy-induced peripheral neuropathy is a painful disorder of the peripheral nervous system that afflicts over half the people with cancer who receive neurotoxic chemotherapy medications (platinum compounds, vinca alkaloids, taxanes and proteasome inhibitors). Chemotherapy-induced peripheral neuropathy is increasing in prevalence due to medicine's success in making cancer curable or a long-term disease.
To understand chemotherapy-induced peripheral neuropathy in depth, Dr. Staff's research team has established a human neuronal culture system derived from iPSCs manufactured from skin biopsies. This culture system allows them to harness the complexity of human biology to more faithfully model chemotherapy-induced peripheral neuropathy in a dish.
Work in the Translational Neuromuscular Disease Research Lab identified two major components of the neuron, microtubules and mitochondria damaged by chemotherapeutic agents. Microtubules provide structure to neurons and allow materials to move up and down the axons; whereas, mitochondria are the primary energy producers of the neuron.
When exposed to a neurotoxic chemotherapeutic agent — bortezomib — microtubules exhibit increased polymerization. Interestingly, taxanes, another neurotoxic chemotherapeutic group with an entirely different cancer-killing mechanism, lead to a similar change in microtubules. Bortezomib also changes the ability of mitochondria to move up and down the axons, preventing the mitochondria from delivering energy to the appropriate region of the neurons. Ongoing work in Dr. Staff's lab aims to dissect these phenomena to find preceding protein pathways that may be able to be targeted for neuroprotection.
Hereditary peripheral neuropathy (Charcot-Marie-Tooth disease)
Mutations in over 100 genes can lead to peripheral neuropathy. Using human sensory neurons derived from people who have hereditary peripheral neuropathy (Charcot-Marie-Tooth disease), Dr. Staff and his research team is investigating how different genetic mutations lead to neuronal damage. Samples are banked in the Regenerative Medicine Biotrust.