Research
-
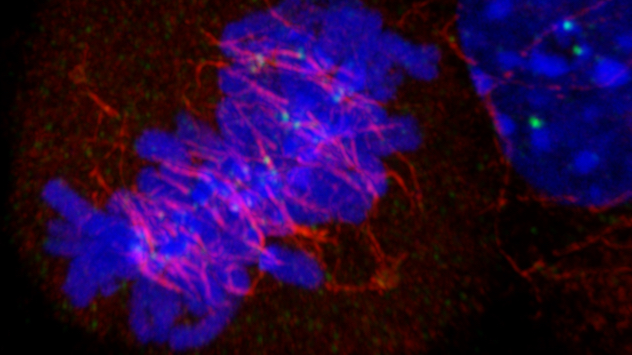
Discovering the regulators of liver cell division
Our approaches have led to insights about the unique regulation of cell division in the liver.
-

Uncovering the genetic basis for liver disease
Dr. Wangensteen's lab uses cutting-edge molecular genetics tools and genomics analysis of patient genetic material to identify genetic drivers and therapeutic targets in liver disease.
Dr. Wangensteen's Genetics of Liver Disease Laboratory is leveraging an in vivo screening paradigm to discover the genes involved in liver regeneration and liver cancer. In addition, the lab is studying inherited variants that are associated with liver cancer development using DNA samples provided by patients. The lab's vision is to develop individualized molecular genetic approaches for treating patients with liver diseases and gastrointestinal cancers.
Hereditary genetics of hepatocellular carcinoma
The lab's team is examining the hereditary genetics of hepatocellular carcinoma by conducting a case-control genetic association study in patients with hepatocellular carcinoma versus matched controls. The goal of this study is to define the germline genetic associations for hepatocellular carcinoma.
Results so far have shown that 11% to 14% of patients with hepatocellular carcinoma harbor pathogenic germline defects in cancer-associated genes. Data also include mutations in DNA repair genes in multiple patients.
The lab is exploring the mechanisms of germline DNA repair deficiencies in driving hepatocellular carcinoma. This investigation is accomplished by examining patient tumors for loss of heterozygosity for these genes.
Dr. Wangensteen's team is investigating whether precision medicine approaches with poly (ADP-ribose) polymerase (PARP) inhibitors might be used in patients with hepatocellular carcinoma. The team also is looking for flaws in DNA repair genes such as BRCA2, BRIP1 and FANCA.
Their study aims to answer these questions:
- What are the clinically meaningful germline mutations linked to hepatocellular carcinoma?
- Are hereditary cancer syndromes more common in people who developed early-onset hepatocellular carcinoma or have a family history of cancer?
- What are the mechanisms of hepatocellular carcinoma arising from defects in homologous recombination?
- What are the implications for targeted therapies of germline gene defects?
- Are there different genetic associations in diverse global populations with hepatocellular carcinoma?
Functional genetic screening
The lab is using a live animal model of liver regeneration and liver cancer to conduct functional genetic screens. The team has developed a model that enables CRISPR-Cas9 screening, which examines up to 1 million hepatocytes in a parallel screening assay. This way, the lab can annotate gene function to address these questions:
- Which genes drive or inhibit liver regenerative responses?
- Which genes regulate hyperproliferation and transformation in the setting of oncogene expression?
- What is the role of gene expression in the cross-talk of proliferating liver cells with an active immune system?
- Which genes regulate energy metabolism in the context of cell division?