Research
Dr. Razidlo's lab team has two main focus areas of research related to cancer metastasis. Our goal is to understand how tumor cells invade and spread through the body, particularly in pancreatic cancers. Our hope is to design new therapies to stop the spread of cancer and improve survival.
Signaling pathways that regulate cytoskeletal remodeling
Actin cytoskeletal dynamics
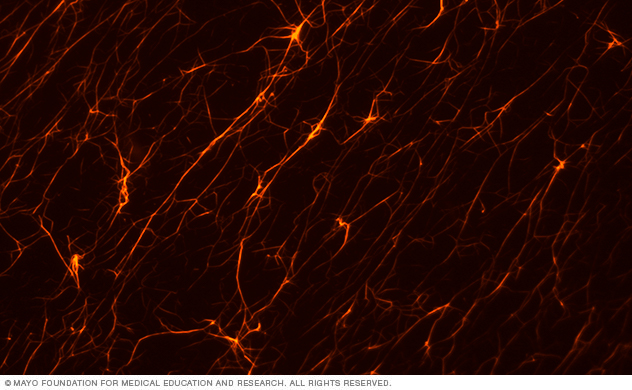
This lab image shows F-actin polymerized in vitro and imaged using fluorescence microscopy.
The dynamic actin cytoskeleton provides the structure for invasive protrusions. We're investigating the proteins that control actin polymerization, branching and bundling, and how they're activated in invasive cancer cells.
Activators of RhoGTPases
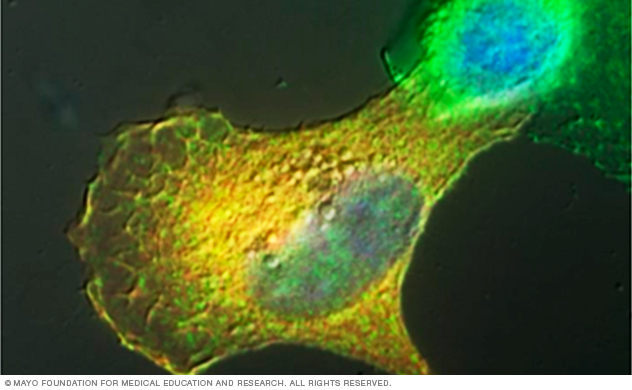
This lab image shows a pancreatic tumor cell ectopically expressing Vav1, an activator of Rac and Cdc42 GTPases. Vav1 expression induces an invasive phenotype.
Actin cytoskeletal dynamics are controlled by the small GTPases Rac, Cdc42 and Rho. These GTPases are molecular switches that are regulated by associated proteins — activation by guanine nucleotide exchange factors (GEFs) and inactivation by guanine nucleotide associated proteins (GAPs).
We're especially interested in how GEFs are aberrantly activated in pancreatic cancers and the consequence for migratory processes. We've described that Vav1, a GEF for Rac and Cdc42 that's turned on in pancreatic cancers, can activate the pro-invasive machinery to upregulate invasive migration. This suggests that Vav1 may also be a potent therapeutic target to inhibit metastasis.
Remodeling of the extracellular matrix
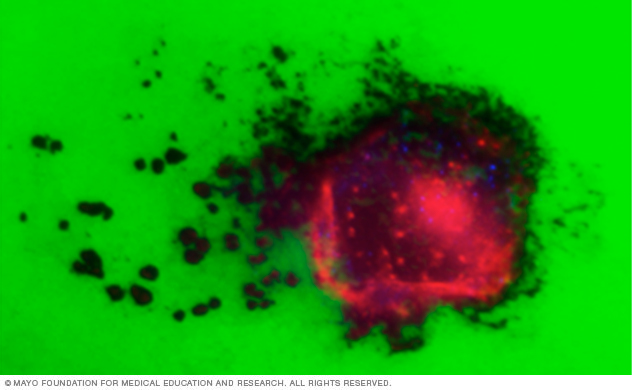
This lab image shows a pancreatic tumor cell (red staining) plated on a green fluorescent extracellular matrix. Invasive structures called invadopodia (red actin puncta) degrade the fluorescent substrate, leading to a loss of fluorescent signal as the cell migrates across the matrix.
Tumor cells form degradative structures called invadopodia that serve as sites for degradation of the extracellular matrix by matrix metalloproteinases. We’re investigating the mechanisms by which tumor cells form these actin-based, degradative protrusions through the concerted action of cytoskeletal, membrane and trafficking dynamics.
We also have found that stromal cells robustly degrade and remodel the extracellular matrix by distinct mechanisms. This emphasizes the need to understand the contributions of the complex tumor microenvironment to design effective therapies to halt pro-invasive ECM remodeling.
Signals from the tumor microenvironment
Cross-talk between tumor cells and the inflammatory microenvironment
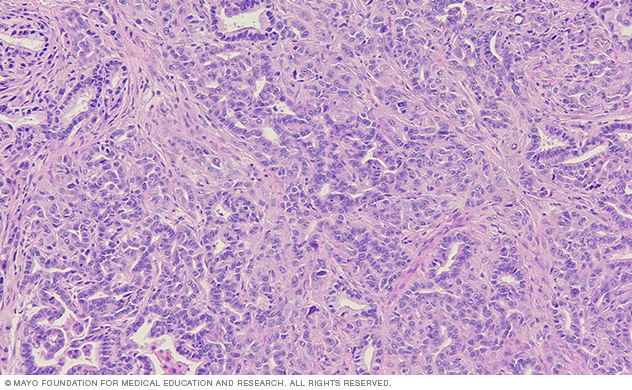
This lab image of a tissue section from a pancreatic tumor shows the ductal tumor cells surrounded by a complex stromal environment.
Inflammatory signals are abundant in pancreatic tumors and can contribute to tumor progression and metastasis. Tumor cells also contribute to the inflammatory microenvironment, illustrating a complex cross-talk among cell types.
We're interested in how the inflammatory environment is modulated in pancreatic cancer and how inflammatory cytokines upregulate the invasive machinery in tumor cells.
Metastatic invasion fueled by excess stromal lipids
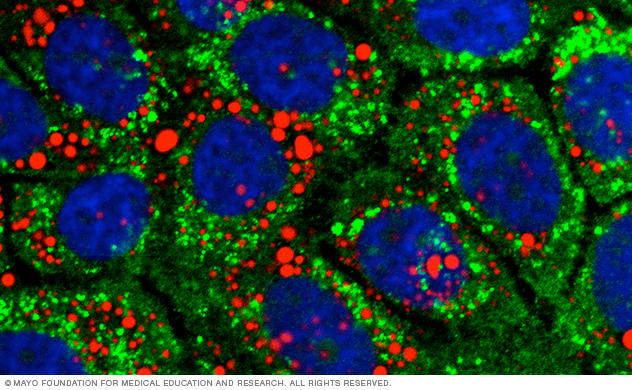
This lab image shows pancreatic tumor cells stained for stored lipids (red) and a degradative compartment (green). Blue indicates the cell nuclei.
Tumor cells accumulate lipids by multiple mechanisms, via increased synthesis and uptake from the microenvironment.
We’re investigating how tumor cells control the balance between lipid storage and catabolism and how stored lipids are subsequently used for invasive migration. We're particularly interested in how this metabolic rewiring is regulated by prominent oncogenes in pancreatic cancer.
Related publications
Research funding
We're grateful for past and present research funding from these organizations: