Myelin Regeneration
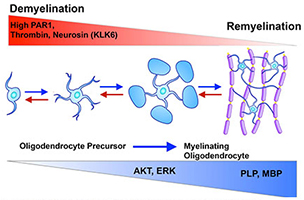
High levels of protease-activated receptor (PAR1) signaling promote demyelination, while reduced levels promote myelin repair by fostering the serine/threonine-specific protein kinase AKT and extracellular signal-regulated kinase signaling. Targeting PAR1 represents a new therapeutic target to promote myelin repair in the developing and adult nervous system.
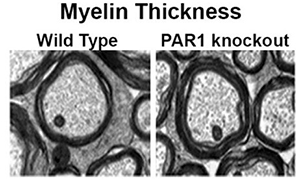
Genetic deletion of PAR1 increases the abundance and thickness of myelin in the adult spinal cord, as cited in Glia, 2015 (Yoon et al.).
Regeneration of myelin in the central nervous system is a crucial area of research. These studies have significant implications for:
- Treating demyelinating diseases such as multiple sclerosis.
- Treating neurodegenerative conditions such as Alzheimer's disease.
- Recovering from spinal cord injuries.
Myelin, the protective sheath around nerve fibers, is essential for the rapid transmission of nerve signals and the health of neurons. When myelin is damaged, it can lead to severe neurological deficits. However, the central nervous system has a limited capacity for myelin repair, which has sparked interest in understanding and enhancing this process.
Recent studies from Dr. Scarisbrick's Neuroregeneration and Neurorehabilitation Laboratory demonstrated that aberrant protease signaling at protease-activated receptor 1 (PAR1), or PAR2, promotes demyelination. PAR1 and PAR2 also actively suppresses the differentiation of oligodendrocyte precursor cells, which are the mediators of both developmental myelination and innate myelin regeneration in adults. The lab's team is investigating the roles of these G protein-coupled receptors in glial biology, including myelination and remyelination, to identify novel targets for therapy to improve functional outcomes.
Related publications
- Sohaei D, Thebault S, Avery LM, Batruch I, Lam B, Xu W, Saadeh RS, Scarisbrick IA, Diamandis EP, Prassas I, Freedman MS. Cerebrospinal fluid camk2a levels at baseline predict long-term progression in multiple sclerosis. Clinical Proteomics. 2023; doi:10.1186/s12014-023-09418-9.
- Ulndreaj A, Sohaei D, Thebault S, Pons-Belda OD, Fernandez-Uriarte A, Campbell C, Cheo D, Stengelin M, Sigal G, Freedman MS, Scarisbrick IA, Prassas I, Diamandis EP. Quantitation of neurofilament light chain protein in serum and cerebrospinal fluid from patients with multiple sclerosis using the MSD R-PLEX NfL assay. Diagnosis. 2023; doi:10.1515/dx-2022-0125.
- Yoon H, Triplet EM, Simon WL, Choi CI, Kleppe LS, De Vita E, Miller AK, Scarisbrick IA. Blocking Kallikrein 6 promotes developmental myelination. Glia. 2022; doi:10.1002/glia.24100.
- Yoon H, Choi CI, Triplet EM, Langley MR, Kleppe LS, Kim HN, Simon WL, Scarisbrick IA. Blocking the thrombin receptor promotes repair of demyelinated lesions in the adult brain. Journal of Neuroscience. 2020; doi:10.1523/JNEUROSCI.2029-19.2019.
- Yoon H, Radulovic M, Walters G, Paulsen AR, Drucker K, Starski P, Wu J, Fairlie DP, Scarisbrick IA. Protease-activated receptor 2 controls myelin development, resiliency and repair. Glia. 2017; doi:10.1002/glia.23215.
- Yoon H, Radulovic M, Drucker KL, Wu J, Scarisbrick IA. The thrombin receptor is a critical extracellular switch controlling myelination. Glia. 2015; doi:10.1002/glia.22788.
- Panos M, Christophi GP, Rodriguez M, Scarisbrick IA. Differential expression of multiple kallikreins in a viral model of multiple sclerosis points to unique roles in the innate and adaptive immune response. Biological Chemistry. 2014; doi:10.1515/hsz-2014-0141.
- Burda JE, Radulovic M, Yoon H, Scarisbrick IA. Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy. Glia. 2013; doi:10.1002/glia.22534.
- Yoon H, Blaber SI, Li W, Scarisbrick IA, Blaber M. Activation profiles of human kallikrein-related peptidases by matrix metalloproteinases. Biological Chemistry. 2013; doi:10.1515/hsz-2012-0249.
- Scarisbrick IA, Radulovic M, Burda JE, Larson N, Blaber SI, Giannini C, Blaber M, Vandell AG. Kallikrein 6 is a novel molecular trigger of astrogliosis. Biological Chemistry. 2012; doi:10.1515/hsz-2011-0241.
- Scarisbrick IA, Yoon H, Panos M, Larson N, Blaber SI, Blaber M, Rodriguez M. Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis. Brain Pathology. 2012; doi:10.1111/j.1750-3639.2012.00577.x.
- Scarisbrick IA, Epstein B, Cloud BA, Yoon H, Wu J, Renner DN, Blaber SI, Blaber M, Vandell AG, Bryson AL. Functional role of kallikrein 6 in regulating immune cell survival. PLoS One. 2011; doi:10.1371/journal.pone.0018376.
- Blaber M, Yoon H, Juliano MA, Scarisbrick IA, Blaber SI. Functional intersection of the kallikrein-related peptidases (KLKs) and thrombostasis axis. Biological Chemistry. 2010; doi:10.1515/BC.2010.024.
- Scarisbrick IA. The multiple sclerosis degradome: Enzymatic cascades in development and progression of central nervous system inflammatory disease. Current Topics in Microbiology and Immunology. 2008; doi:10.1007/978-3-540-73677-6_6.
- Scarisbrick IA, Linbo R, Vandell AG, Keegan M, Blaber SI, Blaber M, Sneve D, Lucchinetti CF, Rodriguez M, Diamandis EP. Kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biological Chemistry. 2008; doi:10.1515/BC.2008.085.
- Vandell AG, Larson N, Laxmikanthan G, Panos M, Blaber SI, Blaber M, Scarisbrick IA. Protease-activated receptor dependent and independent signaling by kallikreins 1 and 6 in CNS neuron and astroglial cell lines. Journal of Neurochemistry. 2008; doi:10.1111/j.1471-4159.2008.05658.x.
- Yoon H, Blaber SI, Evans DM, Trim J, Juliano MA, Scarisbrick IA, Blaber M. Activation profiles of human kallikrein-related peptidases by proteases of the thrombostasis axis. Protein Science. 2008; doi:10.1110/ps.036715.108.
- Angelo PF, Lima AR, Alves FM, Blaber SI, Scarisbrick IA, Blaber M, Juliano L, Juliano MA. Substrate specificity of human kallikrein 6: Salt and glycosaminoglycan activation effects. Journal of Biological Chemistry. 2006; doi:10.1074/jbc.M510096200.
- Oikonomopoulou K, Hansen KK, Saifeddine M, Vergnolle N, Tea I, Blaber M, Blaber SI, Scarisbrick I, Diamandis EP, Hollenberg MD. Kallikrein-mediated cell signalling: Targeting proteinase-activated receptors (PARs). Biological Chemistry. 2006; doi:10.1515/BC.2006.104.
- Scarisbrick IA, Blaber SI, Tingling JT, Rodriguez M, Blaber M, Christophi GP. Potential scope of action of tissue kallikreins in CNS immune-mediated disease. Journal of Neuroimmunology. 2006; doi:10.1016/j.jneuroim.2006.05.022.
- Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6 proteolysis attenuates CNS inflammatory disease. FASEB Journal. 2004; doi:10.1096/fj.03-1212fje.
- Christophi GP, Isackson PJ, Blaber S, Blaber M, Rodriguez M, Scarisbrick IA. Distinct promoters regulate tissue-specific and differential expression of kallikrein 6 in CNS demyelinating disease. Journal of Neurochemistry. 2004; doi:10.1111/j.1471-4159.2004.02826.x
- Scarisbrick IA, Rodriguez M. Hit-hit and hit-run: Viruses in the playing field of multiple sclerosis. Current Neurology and Neuroscience Reports. 2003; doi:10.1007/s11910-003-0087-9.
- Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. Journal of Biological Chemistry. 2002; doi:10.1074/jbc.M202392200.
- Blaber SI, Scarisbrick IA, Bernett MJ, Dhanarajan P, Seavy MA, Jin Y, Schwartz MA, Rodriguez M, Blaber M. Enzymatic properties of rat myelencephalon-specific protease. Biochemistry. 2002; doi:10.1021/bi015781a.
- Scarisbrick IA, Blaber SI, Lucchinetti CF, Genain CP, Blaber M, Rodriguez M. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002; doi:10.1093/brain/awf142.
- Scarisbrick IA, Isackson PJ, Ciric B, Windebank AJ, Rodriguez M. MSP, a trypsin-like serine protease, is abundantly expressed in the human nervous system. Journal of Comparative Neurology. 2001; doi:10.1002/1096-9861(20010312)431:3<347::aid-cne1075>3.0.CO;2-K.
- Scarisbrick IA, Asakura K, Blaber S, Blaber M, Isackson PJ, Bieto T, Rodriguez M, Windebank AJ. Preferential expression of myelencephalon-specific protease by oligodendrocytes of the adult rat spinal cord white matter. Glia. 2000; doi:10.1002/(sici)1098-1136(200005)30:3<219::aid-glia2>3.0.co;2-2.
- Scarisbrick IA, Towner MD, Isackson PJ. Nervous system-specific expression of a novel serine protease: regulation in the adult rat spinal cord by excitotoxic injury. Journal of Neuroscience. 1997; doi:10.1523/JNEUROSCI.17-21-08156.1997.