Mucosal Vaccines
As much as 90% of infections occur at mucosal surfaces, yet the vast majority of effort is directed toward developing and testing vaccines that drive systemic and not mucosal responses. The team believes that mucosal vaccination will be particularly important for protection against HIV-1 and many bioweapons, since mucosal immune responses can provide a critical first line of defense at this key pathogen entry site. Given this, the Virology, Vector and Vaccine Engineering Lab is testing a variety of approaches to maximize antigen delivery to mucosal surfaces.
In one approach, the lab has genetically re-engineered the potent adenovirus type 5 vector to display the mucosal-targeting sigma 1 protein of reovirus type 3 Dearing. This chimeric virus targets both JAM1 and sialic acid present on mucosal surfaces and on M cells in a species-independent fashion.
The team is currently testing the in vivo activity of this vector to enhance gene delivery to mucosal immune sites and decrease problematic gene and vector delivery to cells that do not promote immune response but that produce dangerous side effects. Ad-sigma is being tested as a platform for the delivery of HIV-1, influenza and biodefense antigens to couple with the lab's existing genetic vaccine work. Other approaches include the development of oral capsule vaccines and testing of vectors that target dendritic cells.
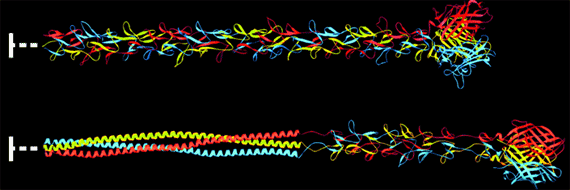
Comparison of the structures of adenovirus fiber and reovirus sigma 1 protein